The expansion of our scientific research to include more GI cancers.
Cambritaxestat is the first autotaxin (ATX) inhibitor to be investigated in cancer patients and is being developed across multiple cancer indications.
iOnctura’s Chief Scientific Officer (CSO), Lars van der Veen, discusses cambritaxestat’s Phase I results and what’s next for the drug.
Q.
What is ATX and why is it an exciting target in oncology?
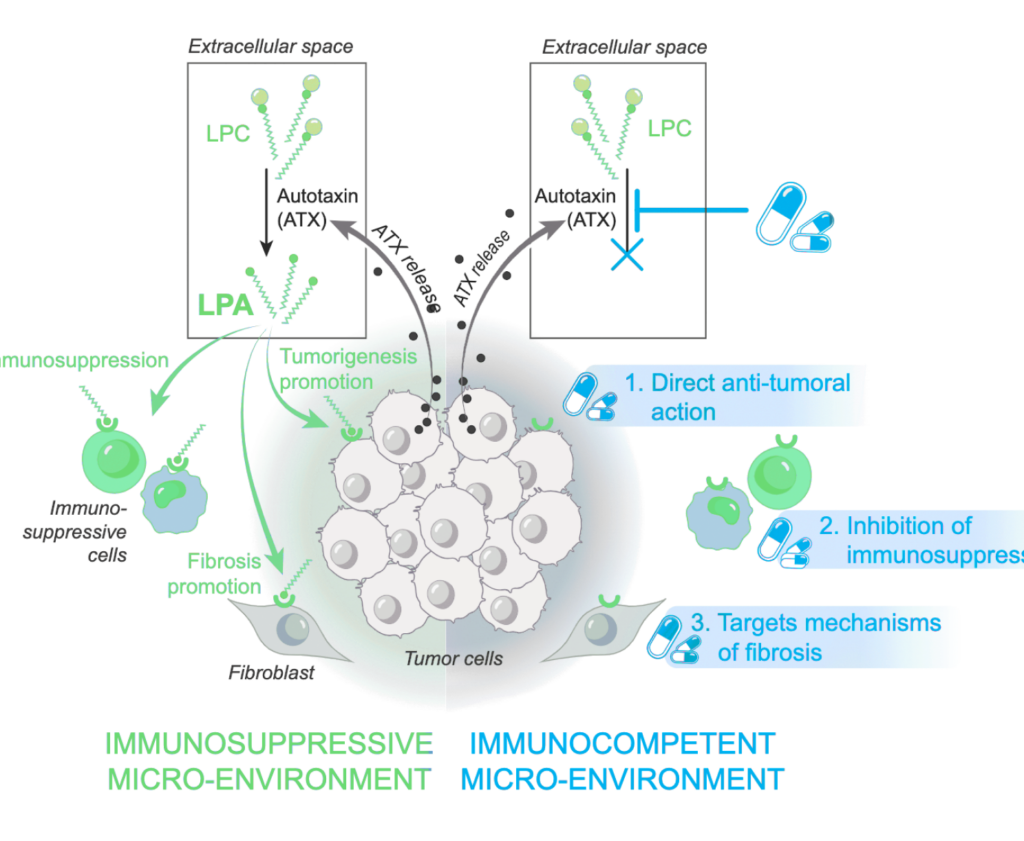
Autotaxin (ATX) is the main enzyme that converts lysophosphatidylcholine into lysophosphatidic acid (LPA), a molecule that drives several cancer-related processes such as cell growth, survival, movement, and fibrosis formation. Because LPA influences cancer in multiple ways, blocking ATX-the source of LPA – can attack cancer cells through several different mechanisms at once. Since this ATX-LPA system is overactive in many types of cancer, targeting ATX could benefit patients across a wide range of tumors.
Q.
Can you summarize the recent news on the Phase I results for cambritaxestat?
The AION-02 Phase I study was the first clinical trial testing an ATX inhibitor in cancer patients. The primary objective was to assess safety and tolerability of escalating doses of cambritaxestat (100mg to 800mg BID) in combination with standard chemotherapy consisting of gemcitabine and nab-paclitaxel (GnP). In 16 patients with metastatic pancreatic cancer (mPDAC), cambritaxestat waswell tolerated in combination with GnP at all dose levels tested; in particular,no dose-limiting toxicities and no cambritaxestat-related serious toxicities were observed. Cambritaxestat showed dose-dependent reduction in ATX-dependent plasma lipids i.e. LPA, confirming robust on-target activity. Furthermore, in an external control study, we found that LPA reduction was not observed in mPDAC patients receiving GnP chemotherapy alone. Although the design of the AION-02 study doesn’t allow to draw firm conclusions on the clinical activity of the individual components of the combination, 10 out of 15 evaluable patients had some degree of tumor shrinkage with two patients having a confirmed partial response at the 200 mg dose level. Interestingly, a more consistent and durable decrease of the tumor marker CA 19-9 was observed starting at the 200 mg dose level suggesting better disease control in the higher dose level cohorts.
Q.
Why is the cambritaxestat data so appealing?
Given that many patients with cancer are treated with a combination of therapies, especially in the first line of therapy, we are encouraged to see these initial results in pancreatic cancer, which show cambritaxestat can safely be combined with a standard of care therapy.
There is a strong biological rationale to suggest it could offer benefit to patients with other gastrointestinal (GI) malignancies as well, and we are therefore excited to explore its potential in other diseases.
Q.
Can you tell us more about this expansion to other GI malignancies?
Like pancreatic cancer, most other GI cancers are associated with high levels of ATX. When we started to explore cambritaxestat in a variety of different GI cancer models, we found that cambritaxestat inhibited the growth of a number of liver cancer cell lines[1]. Our preclinical research program in liver malignancies is currently actively generating data on cambritaxestat to support clinical development.
Q.
What is the scientific rationale to explore cambritaxestat in liver cancer?
ATX overexpression positively correlates with liver fibrosis stage and it also confers an eight-fold higher risk of death from liver cancer. The ATX-LPA axis disrupts lipid homeostasis, increases hepatic stellate cell activation, amplifies fibrotic signaling, promotes liver cancer development and suppresses an anti-cancer immune response.
Preclinical validation demonstrates that hepatocyte-specific ATX deletion protects against liver fibrosis and hepatocellular carcinoma (HCC) development in animal models, while pharmacological ATX inhibition significantly reduces histological fibrosis and inflammation[2].
In animal models, we’ve shown efficacy of targeting ATX in the progressive liver disease, Metabolic Dysfunction-Associated Steatohepatitis (MASH). In MASH, we know that there is fat accumulation in the liver (steatosis), along with inflammation and fibrosis[3].
Disease progression of MASH can lead to the increased risk of primary liver cancers.
We are conducting comprehensive studies in HCC and intrahepatic cholangiocarcinoma (iCCA) models, evaluating combination strategies, and identifying predictive biomarkers.
We aim to present early data at scientific conferences in 2026 and beyond.
Q.
Can you tell us about the medical need for new medicines to treat liver cancer?
Liver malignancies represent a devastating global health challenge. HCC remains the sixth most common malignancy worldwide and third leading cause of cancer death[4].
With more than 900,000 new cases annually, five-year survival rates for unresectable HCC remain below 10%. Only 20-30% of patients are eligible for curative treatments, with cancer recurring 40-80% of the time even after potentially curative interventions[5][6].
iCCA, the second most common primary liver malignancy, presents equally formidable challenges with rising global incidence.
Complete surgical resection remains the only curative option, feasible in fewer than 15-35% of cases, with recurrence rates exceeding 60%[7].
Patients receive checkpoint- and angiogenesis inhibitors and chemotherapy first line but following that, there are no approved therapies and no standard care treatment options.
Q.
What are your development plans for cambritaxestat combinations?
We believe cambritaxestat is an ideal combination partner, both in pancreatic cancer and other GI malignancies. We are interested in exploring intra-portfolio combinations as well as combinations with approved or experimental drugs. We are keeping the patients in mind and how best to serve them with an efficacious and safe drug underpinned by robust data, to extend quality of life for patients.
As we advance toward further clinical testing, we remain committed to combating neglected and hard-to-treat cancers with our autotaxin inhibitor cambritaxestat.
The expansion of our scientific research to include more GI cancers, and specifically liver cancer, represents a natural evolution of our therapeutic strategy, guided by robust scientific rationale and driven by urgent need to improve outcomes for patients facing these devastating diseases.
Sources
[1] Centonze et al. J Exp. Clin. Can. Res. 2024
[2] Erstad et al; Mol Cell Oncol. 2017 Mar 31;4(3):e1311827
[4] World Cancer Research Fund, accessed October 2025
[5] Belghiti & Kianmanesh, HPB 2005;7(1):42–49
[6] Abdelhamed & El-Kassas, Liver Res. 2023 Nov 19;7(4):321–332